Brilliant Invention Gen 20 Hydrogen Generator
Brilliant Invention Gen 20 Hydrogen Generator ---update Feb 7... Full Story
Perth, West Australia

Corrosion of stainless steel due to chloride induction is a common fault in any electrolysis process even using high grade stainless steel . THis process is because of the high solubility of chlorine gas in water and that even though water supplies have been distilled , the Distillation processes commonly used are not good enough to remove all chlorine and that any atmospheric chlorine rapidly dissolves in distilled water open to the atmosphere.
There are very good distillation supplies but they are expensive. Ground water and demineralized water show traces of chlorine, chloride and Hyperchlorite.
There are methods of removing the choline to very low levels so that it does not cause a problem in Hydrogen electrolysis systems — Using a Carbon filter system in the electrolysis system is a good way of cleaning the water. Another method is using our newly patented Ionic Resin filter module to clean the water before adding it to the electrolyte tank. A third way is to use a Specialist anode / anodic coating that resists the anode oxidation reaction or stops it completely due to its oxidation potential.
Below is a document that explains the Process of Corrosion is an electrochemical process leads to surface wastage of metals.
More information about our Hydrogen generation processes can be found from Gavan on 61 403177183 https://hydrogenfuelsystems.com.au and gavan@hfuel.com.au
https://hydrogenfuelsystems.com.au/wp-content/uploads/2025/01/Mechanism-of-Chloride-Induced-Corrosion.docx
Mechanism of Chloride Induced Corrosion (Pitting Corrosion)
February 5, 2017 TeamCivil Concrete, Durability
Mechanism of Chloride Induced Corrosion
Concrete is an alkaline material, having a pore solution of pH ranging between 12-13. The high alkalinity exists due to hydration of cement releasing sodium hydroxide (NaOH) and potassium hydroxide (KOH) in a saturated calcium hydroxide solution (Ca(OH)2). This alkalinity provides the steel with a high degree of protection against corrosion by forming a passive layer on its surface. This layer is dense and impermeable ferric oxide (Fe2O3) film and, if fully established and maintained, it prevents further corrosion of the steel
However, chlorides can cause corrosion of steel even if high alkalinity is maintained >12. Chloride ions do not directly attack the integrity of concrete causing degradation, but they pass through its pores until reaching the steel surface. Chloride ions then react with the oxide film causing a local breakdown – a pit, hence the name pitting corrosion – within it.
Corrosion is an electrochemical process leads to surface wastage of metals. In order for corrosion to occur, four basic elements are required:
1. Anode – site where corrosion occurs and from which current (electrons) flows.
2. Cathode – site where no corrosion occurs and to which current flows.
3. Electrolyte – a medium capable of conducting electric current by ionic current flow. In the case of concrete, the alkaline pore solution constitutes the electrolyte.
4. Metallic path – connection between the anode and cathode, which allows current return and completes the circuit. Steel serves this purpose in reinforced concrete.
The combination of these four elements forms what is known as “corrosion cell”. A schematic diagram for corrosion cell is shown in the following figure.
Schematic corrosion cell
Regarding chloride induced corrosion, areas no longer protected by the passive film at the formed pits act as the anode of the corrosion cell, while the cathodic reaction takes place at the still passive areas. The chemical reactions involved are as follows:

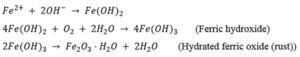
The next figure shows a schematic illustration of the full pitting corrosion process. It is important to mention that the presence of oxygen and moisture is necessary for the precedence of corrosion process. The absence of one of them will terminate the progress of corrosion.
Mechanism of chloride induced corrosion
Views: 25
Wednesday, January 22, 2025Brilliant Invention Gen 20 Hydrogen Generator ---update Feb 7... Full Story
Drop in oil price and Hydrogen. April 22 2020... Full Story
An outlook of hydrogen as an automotive fuel February... Full Story
Designing and Analysis of Hydrogen Power Vehicle along with... Full Story
Application of Hydrogen as Fuel Supplement in Internal Combustion ... Full Story
Payment Methods Partner:

© 2023 - Hydrogenfuelsystems pty ltd