Brilliant Invention Gen 20 Hydrogen Generator
Brilliant Invention Gen 20 Hydrogen Generator Oxy-Hydrogen gas is... Full Story
Perth, West Australia
The electrolysis of water appears simple but is in fact two reactions – 1. An oxidation reaction producing oxygen gas and a reduction reaction producing Hydrogen gas
This can be shown as to half Redox equations with their respective reduction potentials , which shows the minimum applied voltage to produce Hydrogen from water.
An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy [voltage applied between two electrode s; an anode (positively charged electrode) and a cathode (negatively charged electrode)] to drive a chemical reaction that would not otherwise occur.
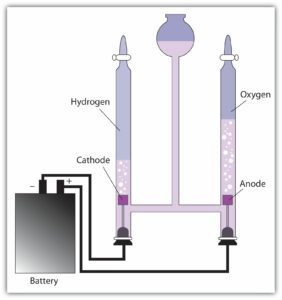


The Reaction producing Oxygen at the anode is
2H2O = O2 + 4H+ + 4e– voltage required E0= -1.23 volt
T raction producing Hydrogen at the Cathode (negative terminal)
4H2O + 4e = 2H2 + 4OH– + 4e– voltage required E0= –0.83volt
The Overall reaction by combining these two half equations is
4H2O + O2 = 2H2 + O2 voltage required E0= – 2.06 volt
As can be seen the minimum voltage to produce Hydrogen gas is 2.06 volts per cell and if you have anything less than this voltage all you will produce is boiling water.
The issue becomes significant in many home designed electrolysis units . Common design neutral plate systems use 100 up to 200 plates to make hydrogen gas by electrolysis and all they end up making is steam.
A 100 plate plate design having 100 cells requires an Input voltage of at least 206 volts , just to equal required RedOx voltage. At a 12 volt input the best they can do is 0.12 volts per cell , and they make the water boil.
As well as the required RedOx voltage required an over voltage of 0.5 volts per cell is required to overcome the internal resistance off the Neutral plate assembly. For a 100 plate assembly that requires another 50 volts from the battery system. Needless to say this cant happen and the cell made is simply heating water, not making Hydrogen.
Another point to consider in producing hydrogen gas is to effectively remove the bubbles from the electrodes fast enough . Simply letting the bubbles rise by Buoyancy results in the free surface area of the plates being reduced for a large percentage of the time, making the cell resistamce higher and needing more voltage or heating the plates making steam. The trick is to match the pressure head of the pump to the pressure lost over the total plate area while still producing laminar fluid flow past the electrodes . Too much pressure produces turbulent flow and reduces gas production rate by increasing resistance. Again this is a property dependent up the plate surface area , plate geometry and plate separation , electrolyte concentration and fluid temperature.
A Detailed calculation involving the variables and the solution diffusion coefficient can be used to design an efficient and high output Hydrogen….. Not the simple defective “Neutral plate units” generally seen on YouTube .
The Systems produced By https://hydrogenfuelsystems.com.au are designed and manufactured with all these variables used to produce a system that is a highly efficient unit that Increases Powerand increases fuel economy. Gavan can be contacted during workinh hours on 0403177183 on email at glknox11@live.com
Send Gavan Your name , email and postal address and contact number and he will send you a quote for a system for your vehicle
Views: 34
Thursday, December 1, 2022Brilliant Invention Gen 20 Hydrogen Generator Oxy-Hydrogen gas is... Full Story
Drop in oil price and Hydrogen. April 22 2020... Full Story
INVESTIGATION TO DETERMINE THE EFFECT OF HHO BOOSTER ON... Full Story
Effect of hydrogen blending on the performance and pollutants... Full Story
Adjustable Power Supply Adjustable Power Supply 0-48V 60A |... Full Story
Payment Methods Partner:


© 2023 - Hydrogenfuelsystems pty ltd